Los Angeles - How can the inner wall of the mouth that is accidentally injured by teeth regenerate without a scar within 72 hours? This mystery that has troubled the medical community for many years now has a revolutionary answer. The research results announced today by a joint team from Cedars-Sinai Medical Center, Stanford School of Medicine, and the University of California, San Francisco (UCSF) have, for the first time, cracked the molecular code of "scarless repair" of the oral mucosa, opening the door to completely changing the treatment model of skin trauma.
This preclinical study, published in Science Translational Medicine on July 3, discovered a cell signaling axis called GAS6-AXL through a mouse model, which acts like an invisible suture and can accurately block the activation of the scar-promoting pathway FAK. When the researchers artificially inhibited the activity of the AXL enzyme, oral wounds actually showed a fibrotic reaction similar to that of the skin; conversely, after activating AXL in skin tissue, the wound surface that originally took several weeks to heal actually showed rapid regeneration like the mouth.

"It's like finding a remote control that turns on or off the speed of healing," said Ophir Klein, MD, co-senior author of the study and executive vice president of Cedars-Sinai Children's Health. "We not only explain why the mouth is better at repairing itself than the skin, but we also demonstrate the feasibility of replicating this ability across tissues."
More than 100 million surgical incisions and trauma patients around the world face scarring every year. The team pointed out that existing therapies only reduce scars, and the discovery of the GAS6-AXL pathway may prevent scar formation from the source. Professor Michael Longaker of Stanford School of Medicine (co-corresponding author) revealed that the team has initiated in vitro validation of human skin tissue, and the next step will be to promote clinical trials of AXL-targeted therapies for burn patients.
It is worth noting that the study used a 3D oral mucosal model constructed from human induced pluripotent stem cells (iPSCs), avoiding the controversy of species differences in animal experiments. As Klein emphasized: "Healing technology from the mouth to the skin may be realized faster than imagined."
As a global leader in regenerative medicine, Cedars-Sinai has led a number of groundbreaking studies in recent years, including 3D printing of biological tissues, gene therapy for rare genetic diseases, etc. This study was supported by a special fund from the National Institutes of Health (NIH).

 English
English عربى
عربى Español
Español русский
русский 中文简体
中文简体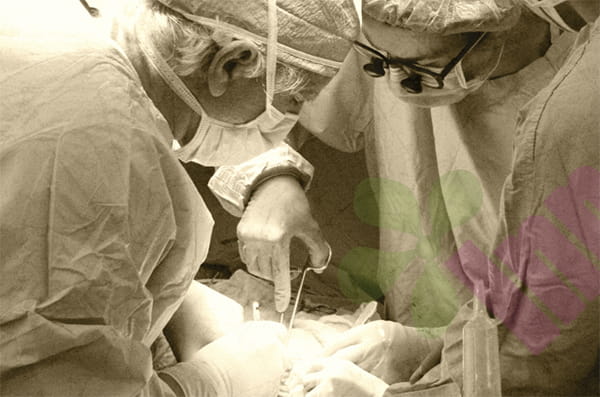











.jpg.png)
.jpg.png)

.jpg.png)


