A research team at the University of California, San Diego School of Medicine has recently revealed a previously unknown mechanism explaining how Staphylococcus aureus—the world's most common pathogen causing skin and soft tissue infections—delays wound healing through bacterial quorum sensing. This discovery provides new insights into developing antibiotic-free wound treatments, potentially reducing the risk of drug-resistant bacteria and improving patient outcomes. The study was published in the *Journal of Clinical Investigation
Despite advancements in wound care techniques, Staphylococcus aureus infections, particularly methicillin-resistant Staphylococcus aureus (MRSA) infections, remain a significant factor contributing to prolonged wound healing and poor patient recovery. MRSA is especially prevalent in hospital settings and is one of the leading causes of surgical site infections, bloodstream infections, and pneumonia.
Using mouse and human wound healing models, the research team discovered that Staphylococcus aureus infection activates its "helper gene regulator (AGR)" system—a quorum sensing mechanism that controls bacterial communication and virulence expression. Once activated, this system significantly inhibits the function of several key metabolic genes in keratinocytes, which are crucial for rebuilding the skin barrier during wound healing.
Further experiments showed that disrupting the Staphylococcus aureus agr system allowed wound healing and keratinocyte function to return to normal, even without a significant reduction in bacterial count. Furthermore, the study found that contact with certain harmless bacteria (such as Staphylococcus aureus) not only did not hinder healing but could actually promote beneficial metabolic activities in skin cells.
Dr. Michelle D. Bagude, a postdoctoral researcher and co-lead author of the study, pointed out: "Our study is the first to clearly define the central role of quorum sensing in Staphylococcus aureus's inhibition of wound healing. Intervening in this mechanism may bypass traditional antibiotics and directly restore the wound's own repair capabilities."
This discovery has significant implications for the prevention and treatment of chronic wounds and nosocomial infections. By developing drugs targeting the agr system, it may be possible to effectively inhibit the pathogenic behavior of Staphylococcus aureus without inducing drug resistance. Simultaneously, the study highlights the crucial role of maintaining a healthy skin microbiome in promoting wound healing.
Professor Richard L. Gallo, Chair of the Department of Dermatology, stated, "This research opens up new avenues for wound treatment. We are shifting from 'killing bacteria' to 'controlling bacteria,' restoring the balance of the wound microecology through precise intervention in bacterial communication systems."
Although this achievement still requires further research and clinical trials before clinical application, it undoubtedly provides an innovative direction for the treatment of chronic, refractory wounds and is expected to drive a paradigm shift in the treatment strategy for infected wounds.

 English
English عربى
عربى Español
Español русский
русский 中文简体
中文简体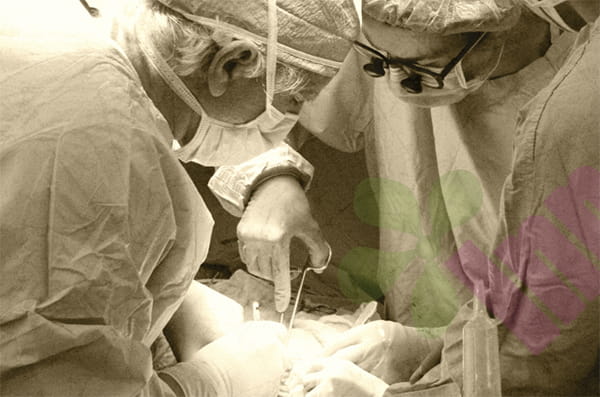











.jpg.png)
.jpg.png)
.jpg.png)


