Research reveals a new breakthrough in BMAC treatment, making personalized repair of ligament and meniscus injuries possible.
Bone marrow aspiration concentrate (BMAC), as a regenerative medicine treatment, has been widely used in recent years for the repair of joint injuries. A recent study published in *ACS Omega* further revealed the differences in the sources of key components in BMAC, providing a scientific basis for future personalized treatment of common sports injuries such as anterior cruciate ligament (ACL), medial collateral ligament (MCL), and meniscus tears.
Bone marrow maceration (BMAC) is a minimally invasive treatment that typically involves extracting bone marrow from the patient (usually from the iliac crest of the hip), concentrating and extracting components rich in stem cells and growth factors, and then injecting this into the damaged area to promote tissue repair, reduce inflammation, and accelerate healing. This therapy can be used independently or in conjunction with surgery and is applicable to various soft tissue injuries such as ACL, MCL, and meniscus tears.
Despite the positive clinical efficacy shown by BMAC, many of its specific components and mechanisms of action remain unknown. Colin Hena, a doctoral student at Lehigh University, points out, "We understand that BMAC has therapeutic potential, but its specific components have long been a 'black box'."
To unravel this mystery, Hena and her team conducted a study, systematically comparing bone marrow samples taken from the hip and shoulder. Traditionally, the hip is considered an ideal collection site due to its rich bone marrow content; however, in some cases, such as during rotator cuff tear treatment, doctors may choose to extract directly from the humeral head of the shoulder to reduce the risk of secondary intrusion. So, does shoulder bone marrow possess similar regenerative potential to hip bone marrow?
The research team used two machine learning models to screen and analyze 109 unique proteins from samples from two different sites. The results showed that although the hip and shoulder bone marrow had a high degree of compositional overlap, the expression ratios of six proteins differed significantly. Hena stated, "These differentially expressed proteins are likely to become key biomarkers for distinguishing between different sampling sites."
This finding suggests that the microenvironment in different bone sites may influence the composition of proteins and growth factors in bone marrow, thereby having a crucial impact on the tissue repair process. Furthermore, the preparation process for BMAC is not yet standardized, and differences in the operation of different extraction kits may lead to varying concentrations of stem cells and proteins in the final product.
Hena emphasized that the machine learning framework developed by the team not only helps in analyzing BMAC components but also provides a method that can be used as a reference for future research on other biological tissues. As further research incorporates variables such as age, gender, and lifestyle, BMAC therapy is expected to move towards truly personalized medicine—doctors can choose the most suitable bone marrow collection site based on the patient's specific situation, thereby optimizing treatment outcomes.
For Hena, who will receive his doctorate in December, this project also highlights the crucial role of data science in contemporary medical research. He stated, "We are living in an age of information overload. In many research fields, the ability to extract insights from massive amounts of data and make informed decisions has become a core capability driving progress."
The advancement of this research has not only deepened the scientific community's understanding of the BMAC mechanism, but also opened up a more precise and efficient development path for regenerative medicine and sports injury treatment.

 English
English عربى
عربى Español
Español русский
русский 中文简体
中文简体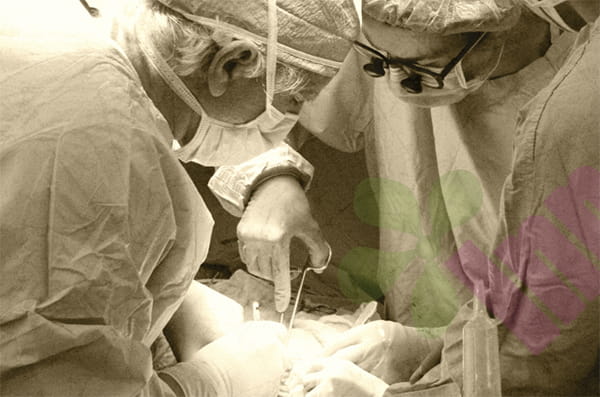












.jpg.png)
.jpg.png)



