A recent study published in Cell Reports Physical Science reveals that Professor Wang Zuankai's team at Hong Kong Polytechnic University, in collaboration with researchers from Sichuan University, has successfully developed a novel material called "ultra-stable mucus-inspired hydrogel." This material has demonstrated excellent gastric damage repair capabilities in animal experiments, and is expected to provide new strategies for the treatment of diseases such as gastric ulcers and gastroesophageal reflux.
Traditional hydrogels such as gelatin, while promoting wound healing and achieving sustained drug release, are easily decomposed in the highly acidic environment of the stomach, limiting their applications. Inspired by natural gastric mucus, the research team constructed an acid-resistant UMIH hydrogel by introducing three key molecular components. Among them, the ELR-IK24 protein neutralizes the local acidic environment, tannic acid enhances the adhesion of the material to the tissue surface, and HDI molecules are responsible for maintaining structural stability under acidic conditions.
Experimental results showed that UMIH exhibited 15 times the adhesion strength of aluminum phosphate gel, a commonly used clinical mucosal protectant, in an acidic environment with a pH of 2, and maintained 50% structural integrity after 7 days, while the control material was completely degraded within 3 days. Furthermore, this material was non-toxic to gastrointestinal cells and effectively inhibited the growth of Escherichia coli and Staphylococcus aureus.
In esophageal injury models in pigs and rats, UMIH can closely adhere to the wound, accelerate tissue repair, reduce inflammatory response, and promote angiogenesis, with efficacy superior to existing clinical standard drugs.
"UMIH exhibits superior adhesion, long-lasting stability, and excellent biocompatibility in acidic environments, providing an innovative solution for gastrointestinal repair," noted Yang Xiao, a co-author of the study from Hong Kong Polytechnic University.
Lou Feng, a collaborator from Sichuan University, stated that the material is low-cost, easy to mass-produce, and the safety of the components has been verified. The future plan is to integrate a drug delivery system with flexible electronic components to develop an intelligent gastrointestinal device that combines treatment and real-time monitoring functions .
Currently, the research team is working to translate this material into clinical applications to further verify its safety and efficacy in humans.

 English
English عربى
عربى Español
Español русский
русский 中文简体
中文简体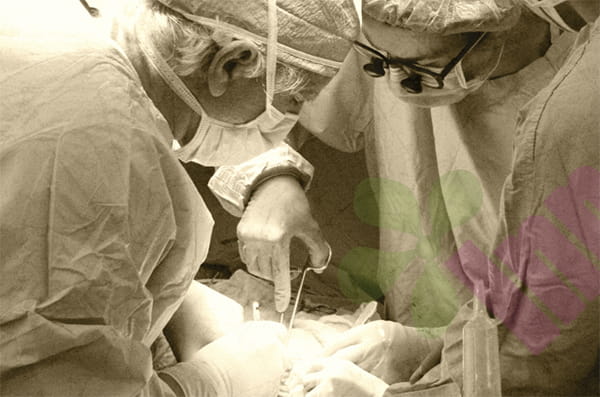











.jpg.png)
.jpg.png)
.jpg.png)


