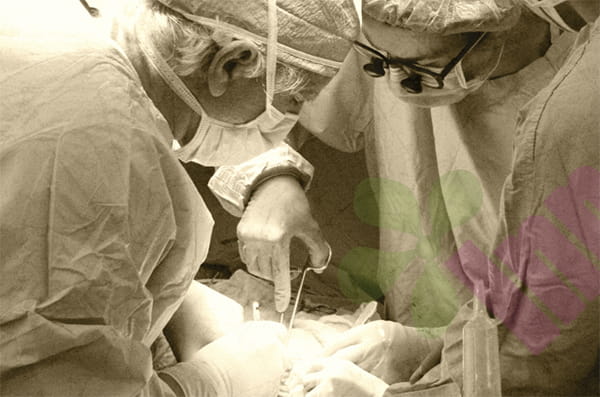
A recent study led by a research team from the University of Arizona has revealed a previously unknown population of circulating immune cells that plays a central role in fibrosis—a pathological process in which excessive accumulation of scar tissue leads to organ dysfunction or disfigurement. This groundbreaking discovery not only deepens our understanding of wound healing mechanisms but also provides important directions for developing new strategies to prevent or treat fibrosis. The findings have been published in the prestigious international journal Nature Biomedical Engineering.
Fibrosis is the common pathological basis for many serious diseases, including pulmonary fibrosis, renal fibrosis, organ transplant rejection, non-alcoholic steatohepatitis, and some heart diseases, and it causes nearly half of all deaths in developed countries. However, the U.S. Food and Drug Administration has not yet approved any treatments that can effectively treat or prevent fibrosis.
“When any organ is damaged, the body initiates a complex healing process, which may lead to the formation of scar tissue. The ensuing fibrosis causes tissue dysfunction and has become one of the leading causes of death in the United States,” noted Karen Chen, associate professor of surgery at the University of Arizona, Tucson School of Medicine and co-first author of the study. “In our study, we identified a specific immune cell circulating in the blood that is one of the important mechanisms driving fibrosis in multiple organs throughout the body.”
The research team discovered through mouse models and in vitro human cell experiments that blocking the signals emitted by these immune cells during wound healing can significantly reduce scar tissue formation. Simultaneously, the expression of these cells also showed an upregulation trend in human fibrotic skin and liver tissue samples.
Wound healing typically involves multiple stages, including hemostasis, inflammation clearance, new cell growth, and tissue remodeling. Abnormalities in this process can easily lead to scar formation. While the roles of inflammation and immune cells in early healing are well-established, their functions in later stages remain less clear.
In collaboration with Professor Chen, Dr. Jeffrey Gertner, Chair of the Department of Surgery at the University of Arizona, Tucson School of Medicine, discovered that myeloid cells (including some immune and inflammatory cells) initiate a series of cellular signals that promote scar formation. Simultaneously, the activity of cells with anti-inflammatory effects decreases accordingly.
The study further demonstrated that disrupting this signaling pathway shifts the cell's repair strategy from "scarring" to "promoting normal healing," and the activity of anti-inflammatory cells is restored. The experimental results showed a significant reduction in fibrosis, and the healed wound tissue was thinner with collagen arrangement more closely resembling normal skin, indicating good tissue remodeling.
This discovery suggests a close interaction between mechanical signals and immune cell regulation during the healing process, which may become a potential therapeutic target for combating fibrosis in the skin and other tissues.
“This research changes our traditional understanding of fibrosis,” Dr. Gertner said. “By targeting immune cells that can sense mechanical forces, circulate in the body, and drive abnormal repair, we have the potential to prevent or even reverse fibrosis in multiple organ systems—from the skin and lungs to the heart and liver.”
The research team also includes Dr. William Hahn, Dr. Mohammad Khreiss, Dr. Maria Gracia Mora Pinos, Dr. Katharina Berryman, Dr. Andrew Hostler, and several student researchers. Their collaborative work lays an important foundation for future translational research in fibrosis treatment.
Source: University of Arizona

 English
English عربى
عربى Español
Español русский
русский 中文简体
中文简体